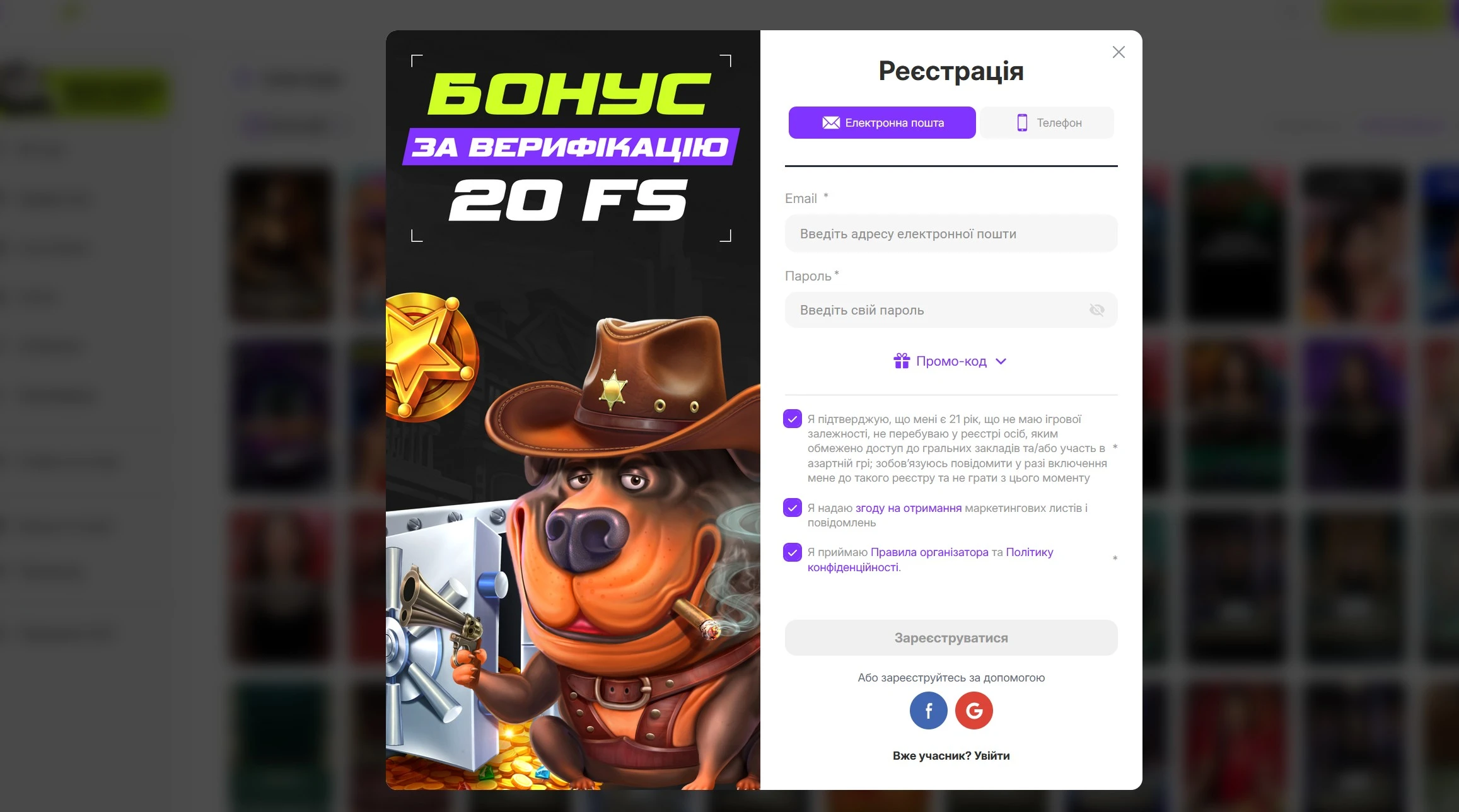
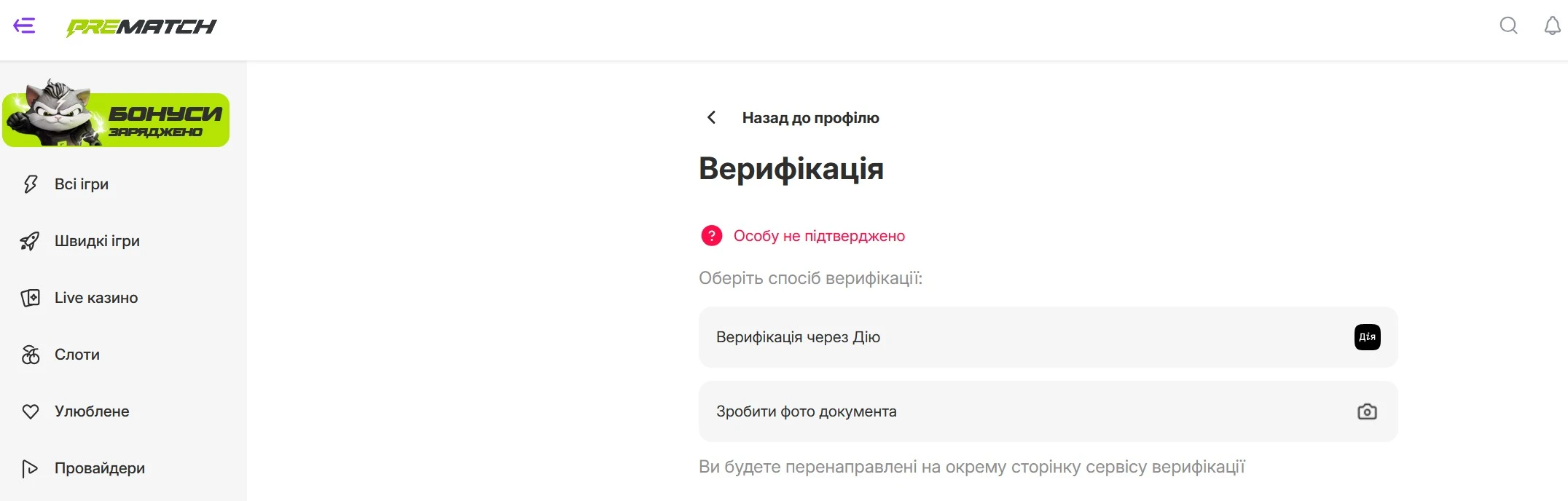
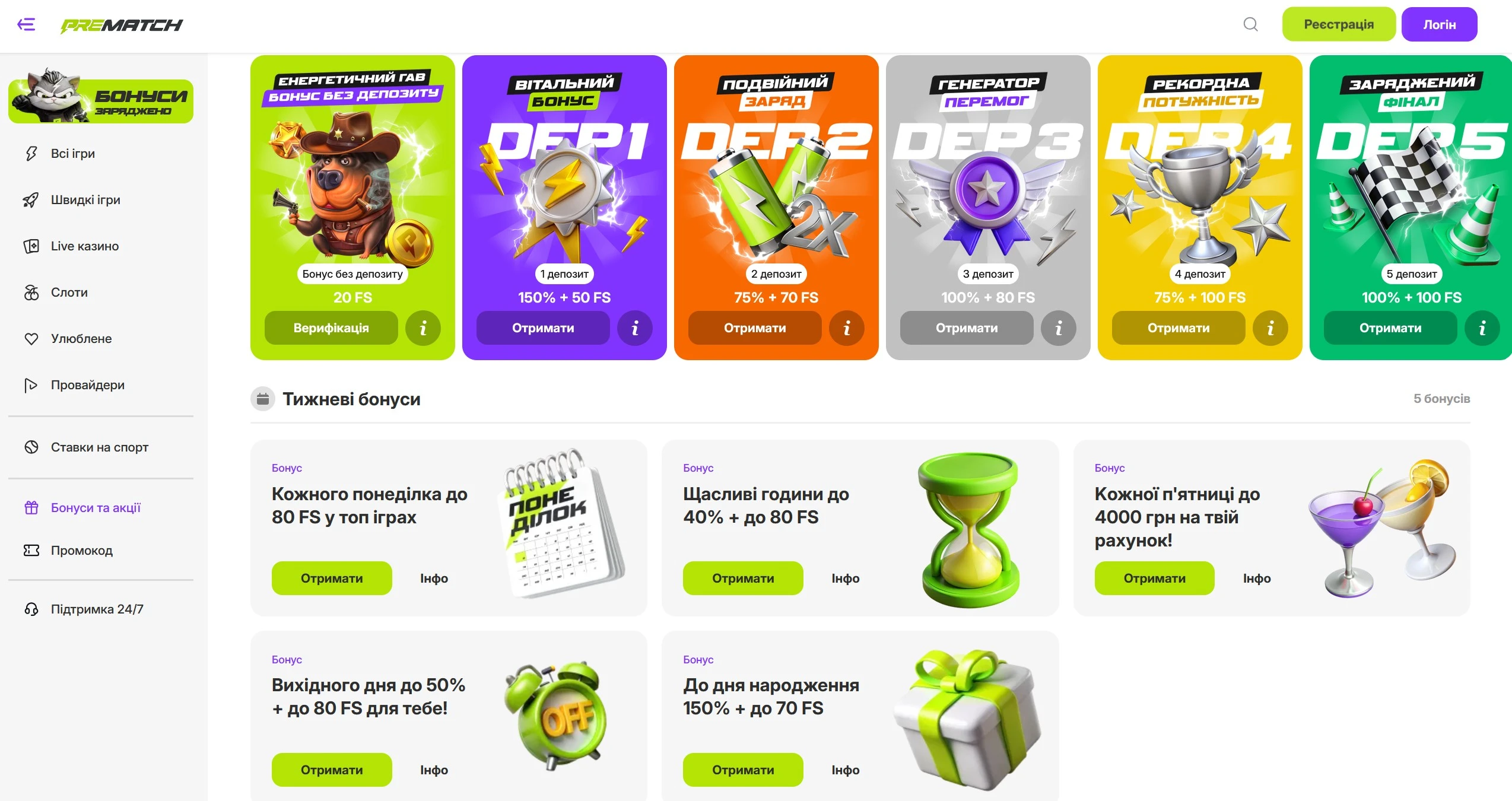
Pre match Casino в Україні
Казино Prematch працює офіційно на підставі ліцензії КРАІЛ №504 від 22.10.2024, виданої ТОВ «ПРЕ МАТЧ». Ліцензія надає право казино легально здійснювати діяльність з організації азартних ігор в мережі Інтернет на території України.